Unveiling a novel immune response in the intestinal epithelium
Researchers from the Jan Dobeš laboratory at Charles University in Prague have made a significant discovery uncovering a novel immune response in the intestinal epithelium. Furthermore, their study delineates a mechanism that controls this immune response.
Their findings were recently published in the Journal of Experimental Medicine in a paper authored by Tomáš Brabec and other members of the laboratory within the Faculty of Science, Charles University. The study was a collaborative effort involving scientists from Martin Schwarzer’s and Dominik Filipp’s laboratories at the Czech Academy of Sciences, as well as Jakub Abramson from the Weizmann Institute in Israel.
The human gut hosts a diverse array of bacteria that play vital roles in digestion, vitamin production, and even the training of our immune system. It has long been established that this gut microbiome is of paramount importance for human well-being. However, it is equally crucial to maintain a tight control over these bacteria to prevent overgrowth, which can lead to inflammatory diseases or other detrimental effects on the body.
The study primarily focuses on a bacterium known as SFB, which is relatively well-studied in the field of immunology. While primarily investigated in mice, SFB is also found in other vertebrates, including humans, albeit in specific contexts. The prevalence of SFB varies and it is influenced by factors such as dietary habits. SFB is particularly present in children and individuals with inflammatory bowel diseases.
The present study focuses on an antigen-specific response, which is one of the key mechanisms of the immune system. According to Dobeš, “antigen-presenting cells play a pivotal role, with MHC molecules acting as a kind of framework to present portions of proteins from sources like bacteria, initiating specific immune responses.”
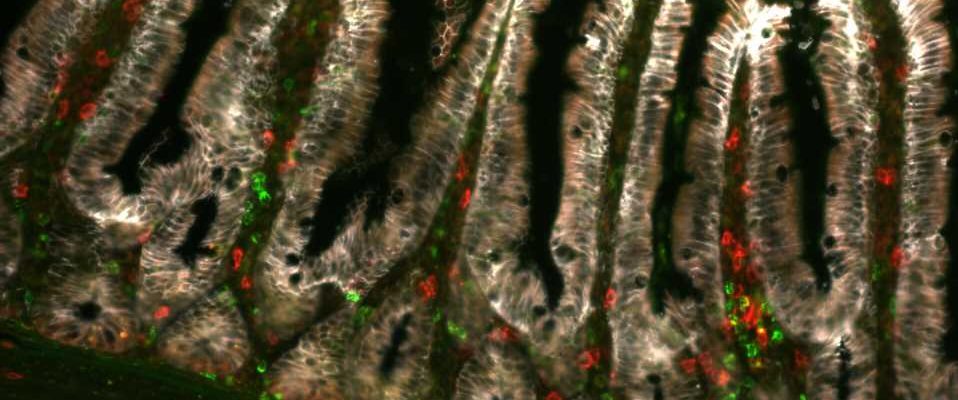
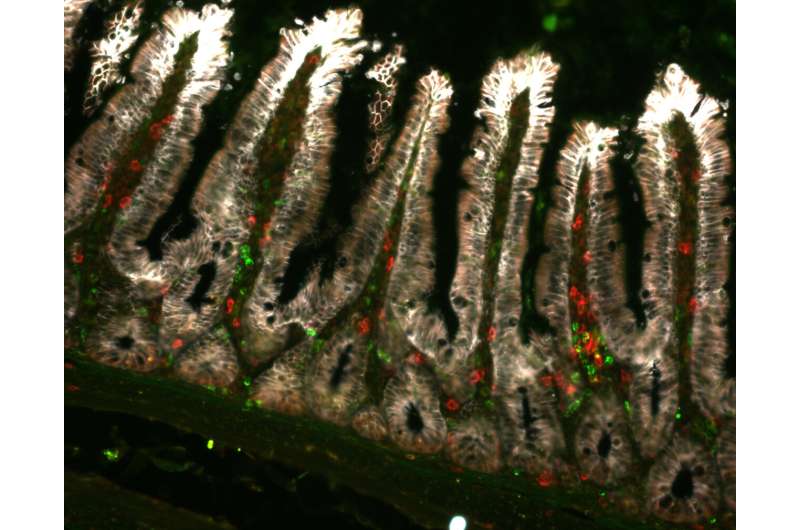
While dendritic cells are well known examples responsible for this function, earlier studies have detected the presence of MHCII molecules on the intestinal epithelial cells. Extraordinary size of the surface area of the intestines allows for a substantial number of MHCII-positive cells, making it somewhat perplexing that their purpose was previously unknown.
“The inspiration for the study emerged in a somewhat humorous fashion, as I and Tomáš Brabec both independently noticed changes in MHCII in the intestinal epithelium,” says Dobeš.
This serendipitous connection eventually led to the collaborative research effort, with the invaluable contribution of Martin Schwarzer’s laboratory, which could provide germ-free mice and specifically colonize them with SFB. Despite initial potentially disappointing findings, says Brabec, “the team persisted, employing sophisticated techniques, including single-cell RNA sequencing, to comprehensively understand cellular activities without the need to focus on previously described immune responses”.
The outcome of their relentless research and utilization of modern methodologies is the identification of a new type of immune response specifically targeting the SFB bacterium. “This response mechanism resembles those used against viruses and involves the generation of granzymes, specific enzymes, in T lymphocytes. Notably, in this scenario, granzymes are expressed in MHCII-activated T cells, contrary to their typical helper function in the immune system”, says Brabec.
In this context, the granzyme-expressing MHCII-activated T cells have become active agents capable of eliminating cells. The study thus introduces a novel immune response and a regulatory mechanism governing the entire system, demonstrating that MHCII in the epithelium, T cells producing granzymes, and the presence of SFB bacteria determine the rate of renewal of the intestinal epithelium.
More information:
Tomáš Brabec et al, Segmented filamentous bacteria–induced epithelial MHCII regulates cognate CD4+ IELs and epithelial turnover, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20230194
Journal information:
Journal of Experimental Medicine
Source: Read Full Article