The risk of an unwanted pregnancy: manufacturer recalls birth control pills
Callback for the anti-baby pill: women could be in spite of taking the drug pregnant
Because the tablets and packaging are incorrectly printed, the pharmaceutical manufacturer Pfizer several batches of an anti-baby pill. The information suggests that the risk of an unwanted pregnancy in spite of taking the drugs.
Not a reliable protection
Fast, safe and convenient: many women rely on hormonal contraceptive methods. No wonder – the anti-baby pill in the proper use and application normally, for reliable protection, and sexual freedom. However, in some preparations, the pharmaceutical company Pfizer, this protection is not necessarily given.
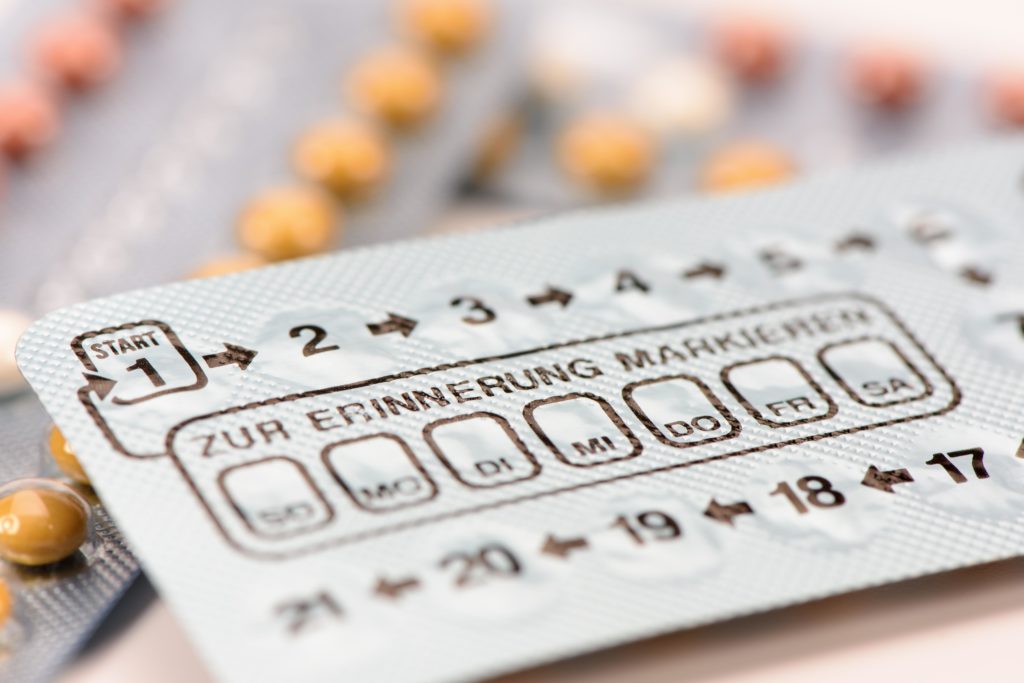
Incorrectly printed taking order
The Landesamt für Gesundheit und Soziales (Lageso) in Berlin informed in a notification of a recall, and a so-called Red-Hand-letter-Pfizer pharmaceutical to the anti-baby pill Trigoa®.
It is noted that a Blister of three batches of this preparation in an incorrectly printed taking order cards on the blisters.
“May result in application errors of the drug is given to the risk of an unwanted pregnancy”, – stated in the message.
Women, the contraceptive Trigoa® of the affected batches X34106, X51153 and W98332 in the period 27.11. – 03.12.2018 have received, are requested, the medicines over the pharmacies to return. (ad)


