PET Imaging Model May Predict Early Dementia in Parkinsons
COPENHAGEN — A prediction model based on PET images acquired within 10 minutes of tracer injection in patients with newly diagnosed Parkinson’s disease may help clinicians identify which patients will go on to develop dementia within 5 years, South Korean researchers report.
Regional cerebral hypoperfusion in brain regions associated with Alzheimer’s disease, identified in these images, “is associated with a higher risk for early dementia conversion in Parkinson’s disease,” said study presenter Seok Jong Chung, MD, PhD, assistant professor, Department of Neurology, Yongin Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea.
As such, early-phase PET scans “can provide additional information on the risk of future cognitive decline in patients with newly diagnosed Parkinson’s disease,” said Chung, who presented the findings here at the International Congress of Parkinson‘s Disease and Movement Disorders (MDS) 2023.
However, a Canadian expert believes that clinicians should not rush to add PET imaging to their workup, noting that clinical features revealed by the patient history and cognitive assessments can predict changes in executive function without the need for imaging.
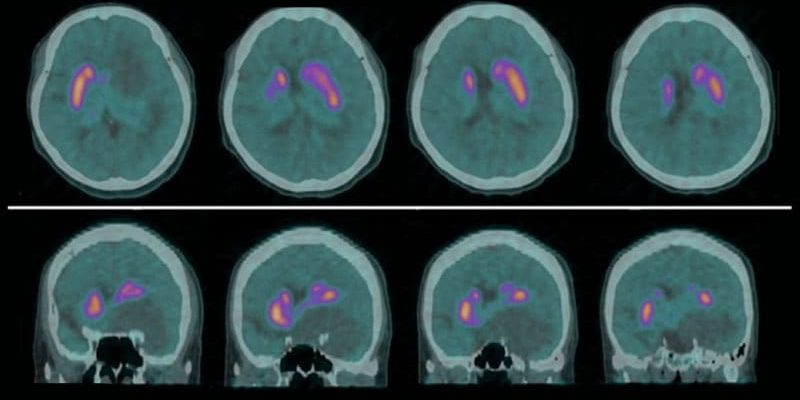
Early-Phase18F-FP-CIT PET
Increased turnover of dopamine terminals in Parkinson’s disease, due to the loss of dopaminergic neurons, can affect the accuracy of standard fluorine-18(18F)-DOPA imaging studies, especially in the early stages of the disease, the researchers note.
To overcome this limitation, 18F-N-(3-fluoropropyl)-2beta-carboxymethoxy-3beta-(4-iodophenyl) nortropane PET (18F-FP-CIT PET) relies on a cocaine analog that binds specifically to striatal dopamine transporter (DAT) sites in the basal ganglia.
Chung told the audience that early-phase 18F-FP-CIT PET images acquired in the first 10 minutes after tracer injection not only resemble standard 2-deoxy-2-[18F] fluoro-D-glucose (18F-FDG) PET images, but also reflect the degree of cerebral perfusion.
So the research team set out to determine whether those images could serve as a predictor of early dementia conversion in patients with newly diagnosed Parkinson’s disease.
They reviewed the medical records of patients with Parkinson’s disease who had undergone both early- and delayed-phase 18F-FP-CIT PET scans on initial diagnosis and had serial cognitive assessments every year during the first 5 years of follow-up.
The early phase 18F-FP-CIT PET scans were qualitatively analyzed, matched to T1-weighted MRI scans, and normalized to the MNI brain template. Standardized uptake value ratios were also determined for each brain region of interest (ROI).
Among 187 patients, 47 experienced Parkinson’s disease with dementia (PDD) conversion within 5 years of initial Parkinson’s diagnosis and were classified as a high-risk dementia group (PDD-H).
A further 15 converted to PDD after the 5-year follow-up, and 125 did not covert to PDD. Together, these 140 patients were classified as a low-risk dementia group (PDD-L).
The researchers showed that patients who were classified as PDD-H were significantly older than those in the PDD-L group, with a mean age of 76.1 years vs 68.8, with a mean age at onset of 74.8 years vs 66.7 years (P < .001 for both).
The PDD-H group also had worse baseline cognitive scores than their low-risk counterparts, in particular on visual memory/visuospatial performance, verbal memory, and attention, working memory and language scores (P < .001 for all).
Comparing tracer uptake in the ROIs in the early phase 18F-FP-CIT PET images, the team found that PDD-H patients had decreased regional uptake vs their PDD-L counterparts, mainly in the temporal and posterior cingulate cortices.
Linear discriminant analysis revealed an ROI-based model based on three regions —the right entorhinal, the left amygdala, and the left isthmus cingulate — could predict the development of PDD within 5 years of diagnosis.
Specifically, the model was able to classify patients as either PDD-H or PDD-L with an area under the receiver operating characteristics curve of 0.837 (95% CI, 0.766 – 0.908).
Chung underlined, however, that the prediction model “should be validated using an external dataset in the future.”
Results Not Particularly Surprising
Approached for comment, session co-chair Jon Stoessl, MD, professor and head of the Division of Neurology at the University of British Columbia in Vancouver, Canada, said the study is from “a very good group.”
He told Medscape Medical News that the results are not particularly surprising, as tracer binding to the dopamine transporter in the early phases of Parkinson’s disease is “likely to represent perfusion,” and the abnormalities revealed are “exactly what you might predict” from what is known about the development of PDD.
The interesting thing about the study is that “many of us don’t use DAT scans on a routine basis,” but in South Korea and other parts of the world “it’s done quite a bit.”
The question is: “If you’re going to be doing that anyhow, can you extract further information from the images? And obviously the answer is, yes you can, and at a relatively low cost,” Stoessl said.
However, he acknowledged that, “honestly, I do very little imaging from a clinical diagnostic perspective…so I’m not sure I could go as far as to suggest myself that people do this.”
Other historical features may predict cognitive impairment, he explained. including age, REM sleep behavior disorder (which is thought to be linked to cognitive function), and of course, overall disease severity.
Stoessl added that clinicians can also “do some very simple bedside cognitive testing; I recommend doing a MoCA [Montreal Cognitive Assessment] on everybody, basically.”
Nevertheless, he stressed that if imaging is being done anyway, “then you might as well try and get more information out of it,” by examining early-phase images for signs of early-onset PDD.
No funding for this study was declared. Chung and Stoessl reported no relevant financial relationships.
International Congress of Parkinson’s Disease and Movement Disorders (MDS) 2023: Abstract 1150. Presented August 28, 2023.
For more Medscape Neurology news, join us on Facebook and X (formerly Twitter)
Source: Read Full Article