Mix and match: New 3-D cell culture model replicates fibrotic elements of pancreatic cancer
Pancreatic cancer is a life-threatening disease with very poor survival rates in patients, and—despite various efforts—its treatment remains challenging. This is because pancreatic cancer is characterized by the presence of ‘fibrosis,’ a pathological scarring process that occurs when the physiological wound healing process goes awry. Thus, to tackle pancreatic cancer, it is crucial to understand the mechanisms driving fibrosis in detail. However, experimental models that are used to study pancreatic cancer have not yet been able to fully replicate the extent of fibrosis in human tissue.
To this end, researchers at Okayama University, Japan, including Professor Mitsunobu R. Kano and Assistant Professor Hiroyoshi Y. Tanaka, found a way to recreate pancreatic cancer tissue “in vitro” (in the laboratory). Using a three-dimensional (3-D) cell culture technique, they devised a method to mix pancreatic cancer cells together with fibrotic components to generate tissues that resemble human pancreatic cancer. Their findings are published in Biomaterials. Prof Kano, who supervised the study, explains, “No cancer is an island entirely of itself. In pancreatic cancer, fibrotic tissue often occupies much more space than the cancer cells themselves. While numerous 3-D models of pancreatic cancer have been reported, ours is the first to allow the tuning of the amount of fibrosis in the model.”
But, why does the presence of fibrosis hinder cancer treatment in the first place? Specifically, the dense and thick fibrotic tissue obstructs the penetration of drugs into the tumor and limits anti-tumor immunity. Assistant Professor Tanaka, who led the study, explains, “With the 5-year survival rate of pancreatic cancer at approximately 9% despite decades of intensive research, a deeper understanding of the mechanisms that underlie fibrosis in pancreatic cancer, as well as its pathophysiological and therapeutic ramifications, is necessary.” Thus, the scientists set out to find ways of understanding fibrotic mechanisms in pancreatic cancer.
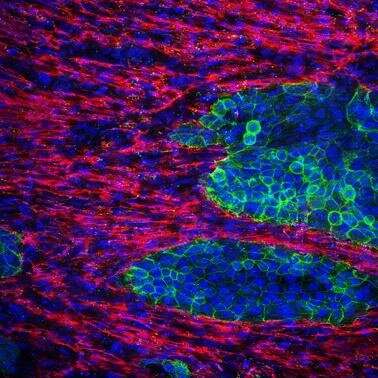
But, this was no easy task, as currently used experimental models of pancreatic cancer, especially in vitro models, fail to replicate the fibrotic components that are seen in pancreatic cancer. Thus, the scientists reasoned that it was important to establish relevant pancreatic cancer models that comprise not only cancer cells but also cells involved in fibrosis. There were other challenges as well: an analysis by the research team revealed that the area occupied by fibrosis in human pancreatic cancer tissues ranges from 40 to 80% among patients. Prof Kano says, “There was considerable heterogeneity among patients, and for our 3-D cell culture model to fully resemble human pancreatic cancer, we wanted to make sure that we could recreate tissue with any given amount of fibrosis.”
Fibrosis consists mainly of cells called “fibroblasts” and other extracellular matrix components secreted by fibroblasts. Considering this, the research team came up with a simple strategy: mix and culture pancreatic cancer cells and fibroblasts at various ratios. “The strategy seemed too simple to work,” Tanaka recollects with a laugh, “But much to our pleasant surprise, it did!” The team successfully showed that by varying the ratio of pancreatic cancer cells seeded against a fixed number of fibroblasts, they could create pancreatic cancer tissues in vitro with different amounts of fibrosis. Importantly, they showed that the area occupied by fibrosis in these tissues can be experimentally tuned to match the amount of fibrosis in the clinically observed range. The team also went on to use the model to successfully understand the molecular mechanisms by which a particular “phenotype” of fibroblasts, characteristic to pancreatic cancer, occurs.
Source: Read Full Article


