Liver fibrosis: Giant cells step in to compensate for impaired immune function
A team of researchers has uncovered a previously unknown compensatory mechanism found in liver disease. If Kupffer cells (KCs), a specific kind of immune cells found in the liver, become impaired by tissue scarring, immune cells originating in the bone marrow flow to the organ, where they form larger cell clusters to perform the same function.
Researchers from the Cumming School of Medicine at the University of Calgary and Charité—Universitätsmedizin Berlin have observed for the first time how the liver preserves its bacterial filtration function even in the presence of disease. Their fundamental findings have been published in the journal Science. They may contribute to developing new treatments for liver damage.
The liver is an amazing organ. The body’s central metabolic organ, it is responsible for both absorbing nutrients and breaking down toxins. It regulates the body’s fat and sugar metabolism and levels of minerals, vitamins, and hormones. Beyond that, the liver plays a lesser known but still vital role as the body’s central immunological organ. The liver is instrumental in keeping the human bloodstream free of pathogens—bacteria, viruses, and fungi. If sepsis, or blood poisoning, occurs, the liver filters out more than 90 percent of the foreign material involved.
This essential function of the organ is performed by a kind of specialized immune cell—macrophages known as Kupffer cells (KCs), which are named for German Baltic anatomist Karl Wilhelm von Kupffer. To perform their filtration function, Kupffer cells are located in the small blood vessels of the liver, the sinusoids, where they receive continuous signals from the hepatic cells themselves and those lining the blood vessels of the liver.
In severe disease, especially chronic liver disease, damage to the liver causes a buildup of scar tissue known as fibrosis, which impairs the organ’s function. In the advanced stage of this tissue remodeling process, the area around the Kupffer cells also undergoes fateful changes—with consequences that were unknown until now.
A research team led by immunologist Dr. Paul Kubes, Ph.D., of the Cumming School of Medicine at the University of Calgary, partnered with colleagues at Charité to investigate this phenomenon. One of their primary aims was to improve treatment options in the future for patients with liver fibrosis. Chronic liver disease is rising sharply around the world. Heavy alcohol consumption and fatty liver disease are the main causes of liver fibrosis and its final stage, cirrhosis.
It is estimated that one in four people worldwide already have fatty liver disease caused by lifestyle factors such as excessive food intake, lack of exercise, and diseases including diabetes and metabolic disorders. Infections and genetic factors can also lead to liver fibrosis. Although there are already good models of liver disease, no one had yet been able to trace the development of liver fibrosis and the key filtration function at the same time.
Role of the immune system in liver fibrosis appears in new light
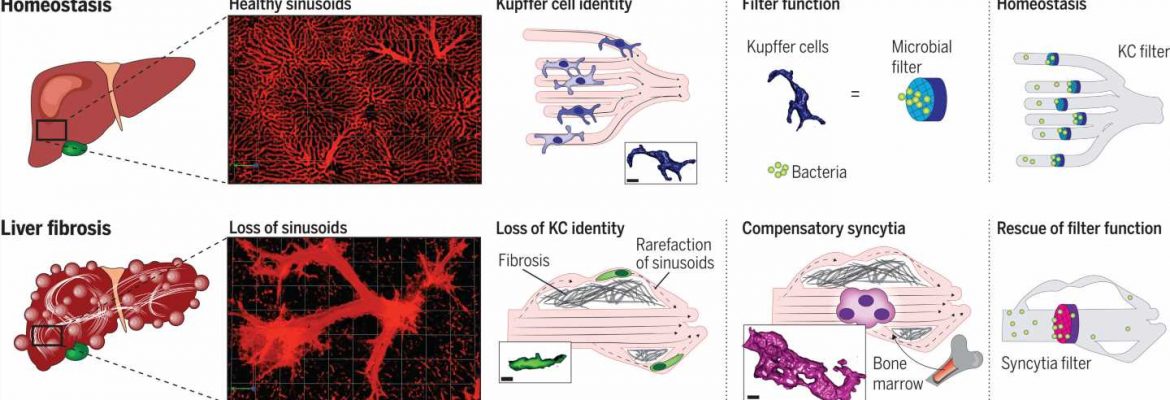
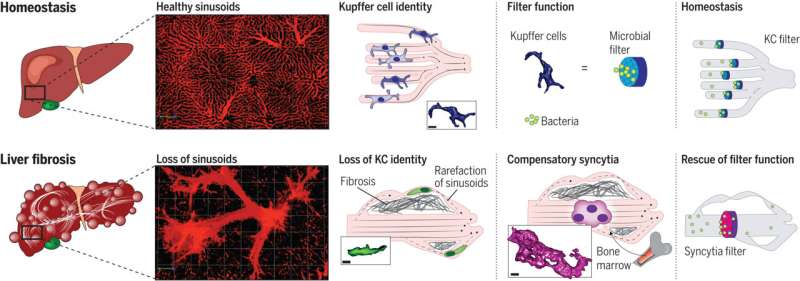
Now, the international team has done just that. Using an innovative microscopy technique that makes it possible to observe cellular functions in detail in a living organism and other microscopy techniques, the researchers closely studied the functioning of Kupffer cells in the animal model and in tissue samples taken from patients with liver cirrhosis. In the process, they identified a new cell type, which they call Kupffer cell–like syncytia.
These are a kind of giant cells—larger, multinucleated clusters of cells formed out of immune cells originating in the bone marrow that have traveled to the scene in response.
Dr. Moritz Peiseler, a scientist and physician at the Department of Hepatology and Gastroenterology at Charité and the first author of the study, describes what takes place during scarring and remodeling of the liver: “More and more liver cells die off. Connective tissue forms all through the organ and around the small blood vessels.”
“The blood is redirected to new, dilated vessels inside and outside the liver. This causes the Kupffer cells to lose contact with their environment, so they end up no longer behaving as if they were in the liver at all. They lose their function, no longer capturing bacteria from the blood, and infections of the bloodstream increase.”
“But it isn’t long before specialized monocytes, immune cells from the bone marrow, infiltrate the liver. They follow the collateral vessels that bypass the previous structures and form clusters large enough to filter out bacteria in the somewhat larger vessels.” This is a life-saving form of compensation triggered by the intestinal microbiome.
The newly formed KC-like syncytia take over the filtration function of the actual Kupffer cells from then on. Since they have to exist inside changed blood vessels, the immune cells that have migrated to the site adapt, forming net-like structures that turn them into an effective microbial filter. The researchers describe the molecular mechanisms involved in these processes in their work.
“These findings change the way we think about the role of the immune system in liver fibrosis,” says Kubes, the head of the study. “Previously, one school of thought was that immune cells from the bone marrow should be prevented from infiltrating the liver. But as our study shows, that could be harmful. Instead of suppressing immune function in advanced disease, it could even be a good idea to promote it.”
Basis for innovative therapies and treatment of liver fibrosis
The study was performed at three large liver transplantation centers. It showed that the processes involved in liver fibrosis in humans are similar to those observed in the animal model. As a result, these discoveries raise fundamental questions for the treatment of patients with fibrotic liver disease.
Infections are a leading cause of death in patients with liver cirrhosis. At the same time, many people with this type of disease experience fibrotic remodeling of the liver, in some cases quite advanced, without increased infections.
“We suspect that the liver preserves its function up to a certain level of damage by recruiting the KC-like syncytia. Ultimately, formation of scar tissue in the liver is also an evolutionarily advantageous mechanism by which a damaged organ ensures survival. So it definitely makes sense for the immune system to adapt as well,” explains Peiseler.
The current study contributes to an improved understanding of how the body’s most important microbial filter functions as liver disease emerges—which could be a basis for developing innovative treatments. Because they are cut off from their environment, the original Kupffer cells no longer act like hepatic immune cells.
This means researchers could investigate how to prevent this loss of identity, which causes loss of function as well. The way the liver responds to pathogenic change is now also known. Supporting this process could help protect patients, since improved microbial filtration function lowers the risk of death from liver cirrhosis and can delay the time of transplantation, which is currently the only treatment option available.
More information:
Moritz Peiseler et al, Kupffer cell–like syncytia replenish resident macrophage function in the fibrotic liver, Science (2023). DOI: 10.1126/science.abq5202
Journal information:
Science
Source: Read Full Article