An unexpected T cell exhaustion factor driving cancer immunotherapy resistance
A research team from the LKS Faculty of Medicine, the University of Hong Kong (HKUMed) has identified an unexpected driver of cancer immunotherapy resistance: the harmful effect of chronic Type I Interferon signaling on tumor-killing CD8+ T cells. These findings provided new insights into the development of exhausted CD8+ T cells, which no longer effectively limit tumor growth, and highlighted a new target for immunotherapy improvement. The research has been published in Cell Reports.
Immunotherapy, whereby drugs reactivate the body’s immune system to fight diseases, is an increasingly popular first-line treatment against various cancers. While numerous cancer patients have benefited from it, the majority are still non-responsive to the therapy, or rapidly develop therapy resistance. The current dismal effective response rate of 20%–30% is the result of many unknown contributing factors.
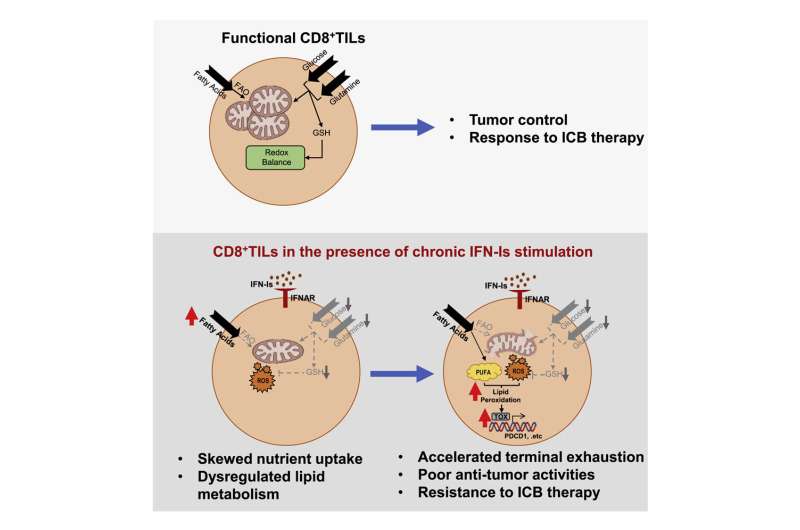
Cancer is usually kept in check by the anti-tumor effector functions of CD8+ T cells. When these cancer-fighting T cells become exhausted (exhausted CD8+ T cells, Tex), the protective response fades away, thus allowing the tumor to undergo immune escape and grow uncontrollably. Preventing and reversing Tex is the primary goal of cancer immunotherapy. However, with the terminal development of Tex resistant to immunotherapy, its efficacy is limited to 20–30%. The research aims to understand the drivers of terminal Tex, and uncover new therapeutic targets to improve cancer immunotherapy.
The research team uncovered a surprising detrimental effect of chronic Type I Interferon (IFN-I) exposure on Tex development and immunotherapy resistance. Cancer patients resistant to immunotherapy also tended to exhibit higher levels of IFN-I signaling.
Through re-analysis of public patient datasets, immune cell culture, and animal models, the research team showed that chronic IFN-I signaling drove CD8+ T cell fate commitment towards terminal exhaustion via promoting lipid peroxidation (LPO), cumulating in therapy resistance.
Dr. Heidi Ling Guang Sheng, Assistant Professor, School of Biomedical Sciences, HKUMed and her team identified that the detrimental IFN-I-LPO pathway can be used to advise patients’ treatment regimens by serving as a biomarker, thus improving the personalization of patient therapy. Moreover, further study of the role of IFN-I opens up avenues for new treatment options to improve patient immunotherapy response.
“These research findings pave the way for new approaches to improve immunotherapy efficacy against cancer. Nevertheless, clinical trials must be conducted to validate the timing, efficacy and safety of IFN-I blockade,” said Dr. Ling.
More information:
Weixin Chen et al, Chronic type I interferon signaling promotes lipid-peroxidation-driven terminal CD8+ T cell exhaustion and curtails anti-PD-1 efficacy, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111647
Journal information:
Cell Reports
Source: Read Full Article