SARS-CoV-2 variants are evolving new ways to evade antibodies and vaccines
Continued replication of severe acute respiratory syndrome-associated coronavirus 2 (SARS-CoV-2) under selective pressure from natural and vaccine-induced immunity has led to the emergence of variants of concern (VOCs) with increased transmissibility or virulence.
These variants acquire mutations in the spike protein receptor-binding domain (RBD) that binds the cellular receptor angiotensin-converting enzyme 2 (ACE2). The RBD and spike protein N-terminal domain (NTD) are targets of neutralizing antibodies.
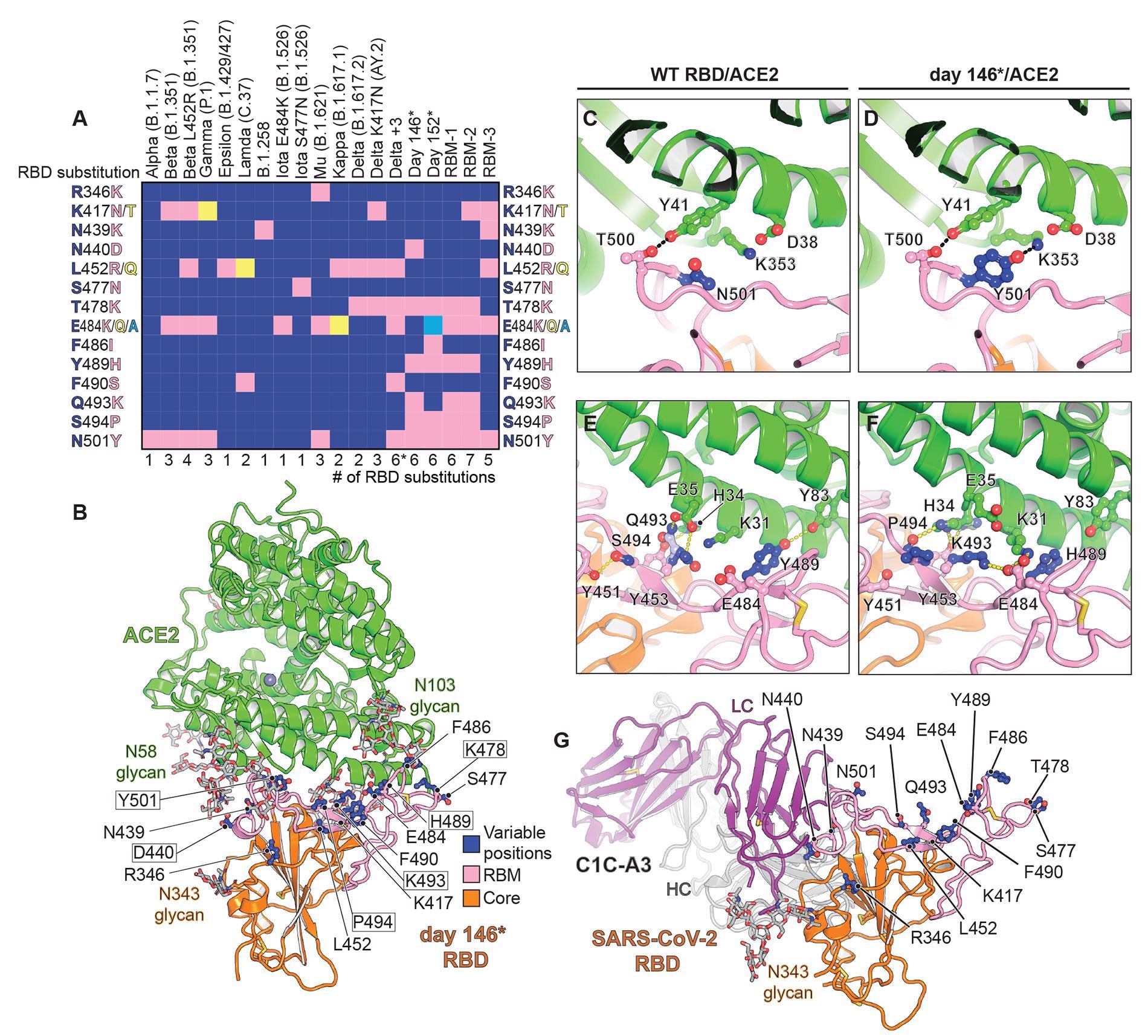
VOCs elicit one to three RBD mutations, which may overlap. For instance, the B.1.617.2 (Delta) variant can acquire the K417NRBD mutation found in the B.1.351 (Beta) variant, generating the Delta AY.2 variant. However, variant monitoring efforts are still under-sampling viral evolution, and an undocumented combination of RBD mutations may have the potential for antibody escape.
The Study
A recent study published in the journal Science investigated the structural pliability of the SARS-CoV-2 spike protein RBD and its capacity to evade neutralizing antibodies.
In this study, monoclonal antibodies were isolated from the blood of a COVID-19 convalescent individual. Venous blood samples were derived from healthy mRNA-1273 and BNT162b2 vaccine recipients.
Findings
The results showed that many RBD mutations occur in a persistently infected immunocompromised host. Furthermore, structural plasticity at the ACE2-RBD interface facilitates the accumulation of such mutations.
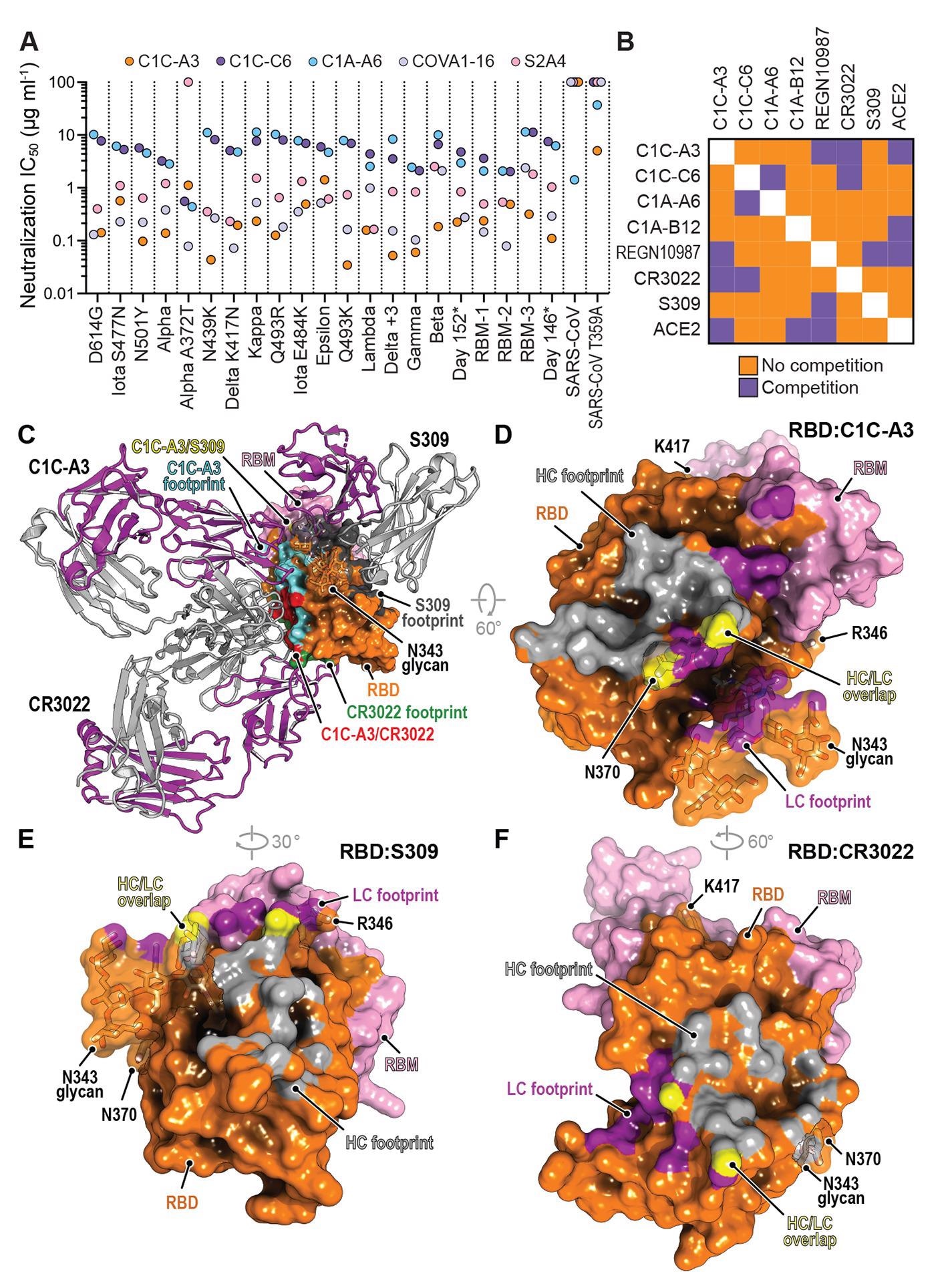
Thus, the phenotypes indicate that further evolved variants were likely to be more elusive to therapeutic antibody neutralization than currently circulating VOCs – and could prove to be resistant to two-component antibody cocktails.
It was observed that after two messenger ribonucleic acid (mRNA) vaccine immunizations and seven days after the second dose, all mRNA vaccine recipients had detectable neutralizing activity against pseudotypes containing an NTD supersite deletion and RBDs with six to seven mutations. However, precise epitopes targeted by this residual neutralizing activity are yet to be identified.
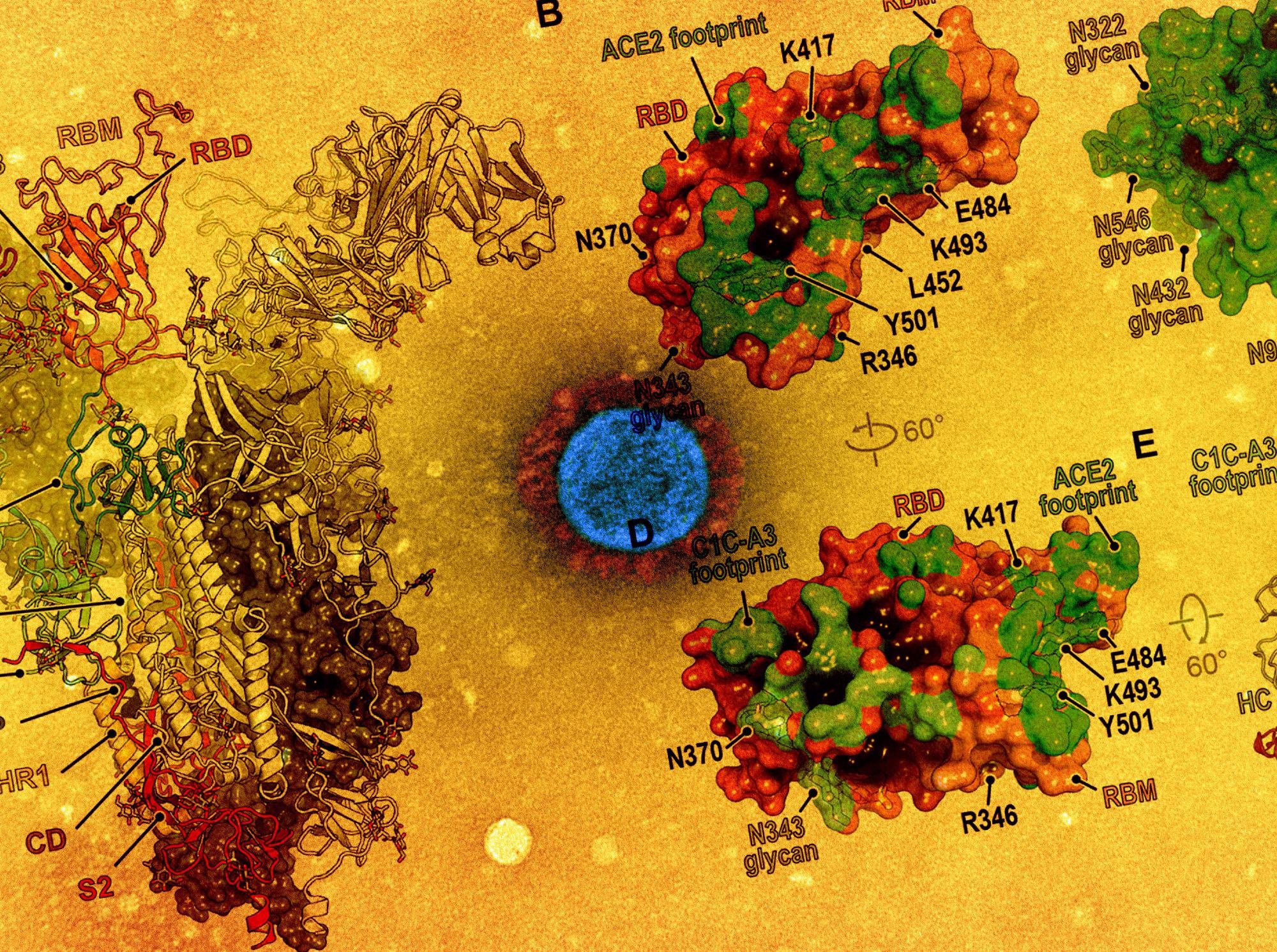
Antibodies targeting the RBD core is speculated to be responsible for the residual vaccine-elicited serum neutralizing activity. The receptor-binding motif (RBM) is a critical site of potent neutralizing antibody binding that is the most antibody accessible and the least masked by glycan and conformational shielding. Hence, continued RBM evolution may modulate antibody responses toward more conserved neutralizing epitopes on the RBD core.
Comparison of immune evasion revealed that variants with an NTD supersite deletion and an E484RBD substitution are the most concerning with respect to resistance to polyclonal antibodies. In fact, E484RBD acts as a significant driver in neutralization escape; furthermore, the pseudotype – Beta, more robustly escapes antibody neutralization than Gamma.
Here, D364NRBD was identified as an additional mutation that would introduce a putative N-linked glycosylation site. This independent acquisition on the same surface of the RBD core suggests that this region may be a target of immune selective pressure.
It was stated that the addition of the N370RBD glycan might be associated with reduced virulence, but chances of secondary mutations nevertheless, which may again restore the viral fitness. Such compensatory mutations would promote ACE2 binding and RBD opening.
In summary, viral replication in human hosts under antibody selective pressure will continue to cause further mutations and newer VOCs. Therefore, future strategies to tackle the ongoing pandemic must involve aggressive variant monitoring efforts, designing next-generation antibody-based therapeutics, updating mRNA- or DNA-based vaccines to rapidly adapt to new variants, and examining the consequences of further viral evolution prior to the emergence of the next highly antibody resistant strain.
- Nabel, K. G., Clark, S. A., Shankar, S., et al. (2021), “Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain”, Science, doi: 10.1126/science.abl6251, https://www.science.org/doi/10.1126/science.abl6251?utm_campaign=SciMag
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Blood, Coronavirus, Coronavirus Disease COVID-19, DNA, Enzyme, Evolution, Glycan, Glycosylation, immunity, Mutation, Pandemic, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Vaccine, X-Ray

Written by
Nidhi Saha
I am a medical content writer and editor. My interests lie in public health awareness and medical communication. I have worked as a clinical dentist and as a consultant research writer in an Indian medical publishing house. It is my constant endeavor is to update knowledge on newer treatment modalities relating to various medical fields. I have also aided in proofreading and publication of manuscripts in accredited medical journals. I like to sketch, read and listen to music in my leisure time.
Source: Read Full Article
