Takhzyro
NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
TAKHZYRO VIAL CMI V3
This medicine is subject to additional monitoring. This will allow quick identificationof new safety information. You can help by reporting any side effects you may get.You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
lanadelumab
Consumer Medicine Information
What is in this leaflet
Please read this leaflet before you start using TAKHZYRO.
This leaflet answers some common questions about TAKHZYRO. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
TAKHZYRO is a new medicine. Please check with your doctor whether there is any additional information about this medicine that you should know since you were last treated with this medicine.
All medicines have risks and benefits. Your doctor has weighed the risks of you using TAKHZYRO against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What TAKHZYRO is used for
TAKHZYRO is a medicine used in adults, and adolescents aged 12 years and older to prevent angioedema attacks in hereditary angioedema (HAE).
You may still experience acute HAE attacks whilst using TAKHZYRO. You should make sure that you carry acute attack treatment and that you use this treatment as advised by your doctor.
HAE is a hereditary condition which means it can run in families. It happens when your blood does not have enough of a protein called ‘C1 esterase inhibitor’ (or ‘C1 inhibitor’), or it does not work properly. This leads to too much ‘plasma kallikrein’, which in turn produces higher levels of ‘bradykinin’ in your bloodstream. Too much bradykinin leads to symptoms of HAE like swelling and pain.
TAKHZYRO contains the active substance lanadelumab. It is a type of protein that blocks the activity of plasma kallikrein. This helps to reduce the amount of bradykinin in your bloodstream and prevents symptoms associated with HAE.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor’s prescription.
Before you use TAKHZYRO
When you must not use it
Do not use TAKHZYRO if you have an allergy to:
any medicine containing lanadelumab
any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin.
It is important to be able to tell when you might be having an allergic reaction as the symptoms are very similar to those of an attack of HAE, so you should discuss this with your doctor.
Do not give this medicine to a person below 12 years of age.
The benefits and risks in children below 12 years of age have not been established.
Do not use this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding.
There is no information on the safety of TAKHZYRO use during pregnancy or breastfeeding. Your doctor can discuss with you the risks and benefits involved.
Tell your doctor if you are using TAKHZYRO before you have laboratory tests to measure how well your blood is clotting.
TAKHZYRO in the blood may interfere with some laboratory tests, leading to inaccurate results.
If you have not told your doctor about any of the above, tell him/her before you start using TAKHZYRO.
Taking other medicines
Tell your doctor or pharmacist if you are using any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
TAKHZYRO may affect other medicines or be affected by them. Your doctor and pharmacist have more information on medicines to be careful with or avoid while using this medicine.
How to use TAKHZYRO
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor, pharmacist or nurse for help.
How much to use
The recommended starting dose is 300 mg every 2 weeks. If you have not had an attack for a long period, your doctor may change the dose to 300 mg every 4 weeks, especially if you have a low body weight.
If you are not sure how much and how frequently you need to inject TAKHZYRO, ask your doctor, pharmacist or nurse.
How to use it
TAKHZYRO should be injected under the skin (subcutaneously).
The injection can be self-administered or given by another person, for example your carer, your doctor, his/her assistant or your nurse.
If you are injecting the medicine yourself, you must receive adequate training by your doctor or nurse. You will find detailed instructions for injections at the end of this leaflet.
How long to use it
Continue using this medicine for as long as your doctor prescribes it for you.
Do not stop using this medicine without consulting your doctor, as a sudden stop may not control your symptoms well.
If you forget to use it
If you forget to use this medicine (or cannot inject it at your usual time), use it as soon as possible but there must be at least 10 days between each dose.
Do not inject a double dose to make up for the dose that you missed.
If you have trouble remembering to use your medicine, ask your pharmacist or nurse for some hints.
If you use too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (Australia: 13 11 26; New Zealand: 0800 POISON or 0800 764766) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have used too much TAKHZYRO. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are using TAKHZYRO
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using TAKHZYRO.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
If you become pregnant while using this medicine, tell your doctor immediately.
It is strongly recommended that every time you inject a dose of TAKHZYRO, write down the date and batch number of the medicine. This is so that you keep a record of the batches used in case you have a severe allergic reaction to TAKHZYRO.
Things you must not do
Do not use TAKHZYRO to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop using your medicine or change the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how TAKHZYRO affects you.
Do not drive or use machines if you feel dizzy after using TAKHZYRO.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using TAKHZYRO.
Like all medicines, TAKHZYRO can cause some side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
reactions where the injection is given – symptoms include: pain, skin redness, bruising, discomfort, swelling, bleeding, itching, hardening of skin, tingling, warmth and rash
dizziness, feeling faint
muscle pain
raised skin rash
allergic reactions including itching, discomfort, tingling of the tongue
abnormal liver-related blood test results.
The above list includes the more common side effects of your medicine.
Contact your doctor or go to the Accident and Emergency at the nearest hospital immediately if any of the following occurs:
allergic reaction which may result in shortness of breath; wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; or rash, itching or hives on the skin.
Tell your doctor, pharmacist or nurse if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using TAKHZYRO
Storage
Keep your medicine in the original carton to protect the vial from light until it is time to use it.
If you take the medicine out of the pack it may not keep well.
Keep the medicine in the refrigerator at 2°C to 8°C. Do not freeze it.
Vials removed from refrigeration should be stored unopened, below 25°C, and used within 14 days or returned to refrigeration unopened until use. Total period stored out of refrigeration, below 25°C, should not exceed 14 days.
Do not shake TAKHZYRO.
Do not store TAKHZYRO or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What TAKHZYRO looks like
TAKHZYRO, a ready-to-use solution for injection under the skin (subcutaneous injection), is supplied in a single-use, glass vial.
Each vial of TAKHZYRO contains 300 mg of lanadelumab in 2 mL solution.
TAKHZYRO solution is colourless to slightly yellow in colour.
Each pack contains one vial of TAKHZYRO.
Each pack also contains one 3 mL syringe, one 18 gauge needle, and one 27 gauge needle (see ‘Instructions for administering TAKHZYRO’ below).
Ingredients
Active ingredient: lanadelumab
Other ingredients:
dibasic sodium phosphate dihydrate
citric acid monohydrate
histidine
sodium chloride
polysorbate 80
water for injections
Sponsor
Australia:
Takeda Pharmaceuticals Australia Pty Ltd
Level 39, 225 George Street
Sydney NSW 2000
Australia
Telephone: 1800 012 612
www.takeda.com/en-au
New Zealand:
Takeda New Zealand Limited
Level 10, 21 Queen Street
Auckland 1010
New Zealand
Telephone: 0508 169 077
www.takeda.com/en-au
This leaflet was prepared in November 2020.
Australian Registration Number:
300 mg/2 mL vial: AUST R 302300
TAKHZYRO is a registered trademark of Dyax Corp.
TAKEDA and the TAKEDA Logo are registered trademarks of Takeda Pharmaceutical Company Limited.
Instructions for administering TAKHZYRO
Important information
Read the leaflet carefully before using TAKHZYRO.
TAKHZYRO is for injection under the skin (subcutaneous injection). Do not inject it into a vein or muscle.
Do not use the vial if the:
medicine is discoloured
medicine contains particles
cap covering the stopper is missing.
Dispose of all needles and syringes in a sharps disposal container.
Materials needed for preparing and injecting TAKHZYRO:
one (1) vial of TAKHZYRO solution
one (1) empty 3 mL injection syringe
one (1) 18 gauge blunt tip vial access needle (to draw the medicine from the vial into the syringe)
one (1) 27 gauge ½-inch pointed tip injection needle (to inject the medicine under the skin)
alcohol wipes
a puncture-proof container for safe disposal of the used syringes and needles.
1. Prepare the vial
1.1 Take the vial out of the refrigerator 15 minutes before use and allow it to reach room temperature before preparing an injection.
Important: TAKHZYRO should be administered within 2 hours of preparing the dosing syringe at room temperature. After the dosing syringe is prepared, it can be refrigerated at 2°C to 8°C and must be used within 8 hours.
1.2 Clean your work area and wash your hands prior to preparing your dose. Do not touch any surface or body part, especially your face, after washing your hands before injection.
1.3 Gather your TAKHZYRO vial and materials and place them on your well-lighted work surface.
1.4 Remove the vial from the packaging. Do not use the vial if the cap covering the stopper is missing.
1.5 Gently turn the vial upside down 3 to 5 times to ensure the solution is mixed.
Important: Do not shake the vial to avoid foaming.
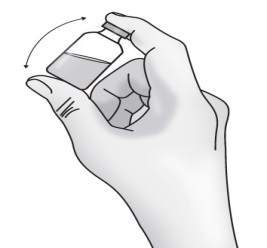
1.6 Inspect the solution in the vial for visible particles or a change in the colour (normally colourless to slightly yellow). Do not use the vial if you see particles or a change of colour.
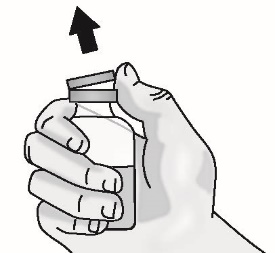
1.7 Remove the plastic cap from the vial. Do not remove the vial rubber stopper.
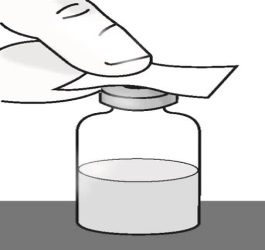
1.8 Place the vial on a flat surface. Clean the vial rubber stopper with an alcohol wipe and allow it to dry.
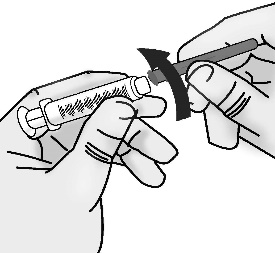
2. Attach blunt tip vial access needle to syringe
2.1 Screw the 18 gauge blunt tip access needle to the 3 mL syringe.
Important: Do not remove the needle cap from the needle when attaching to the syringe.
2.2 Pull back the plunger to fill the syringe with air equal to the amount of solution in the vial (2 mL for the 300 mg dose).
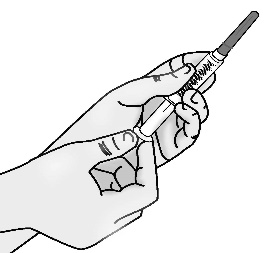
2.3 Pull off the needle cap straight away from the syringe without touching the needle. Do not pull on the plunger.
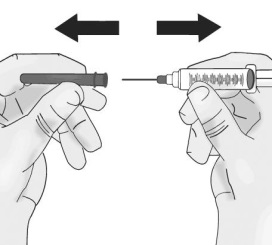
3. Transfer TAKHZYRO into syringe and switch to the pointed tip injection needle
(a) Transfer TAKHZYRO into syringe
3.1 Insert the needle into the centre of the vial rubber stopper.
3.2 Push the plunger down to inject air into the vial and hold the plunger down.
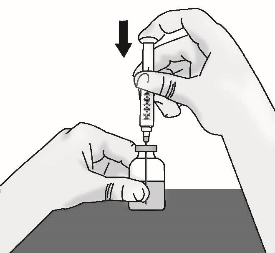
3.3 Slowly turn the vial upside down with needle and syringe attached. Pull back on the plunger to withdraw the full dose in the vial.
Important: Be sure to keep the tip of the needle in the solution to avoid drawing air in as you pull back the plunger.
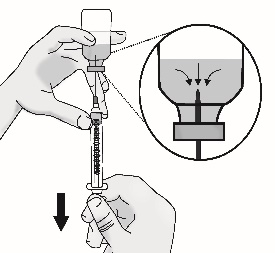
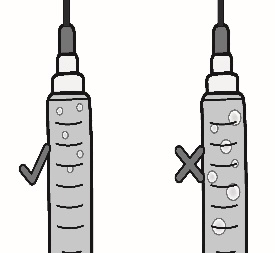
3.4 Remove large air bubbles by gently tapping on the syringe with your fingers until the bubbles rise to the top of the syringe.
Slowly push the plunger, allowing air to go back into the vial, until the solution reaches the top of the syringe.
Repeat this step until large air bubbles are removed.
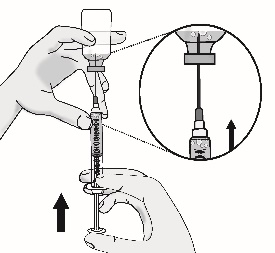
(b) Switch to the pointed tip injection needle
3.5 Without removing the needle from the vial, unscrew the syringe by holding the needle hub and turning the syringe counter-clockwise.
Return the syringe to an upright position as in Step 3.4 above.
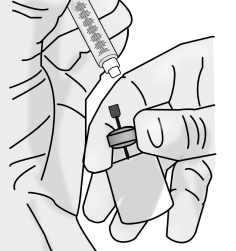
3.6 Discard the 18 gauge blunt tip vial access needle and the vial in the sharps disposal container.
Important: Do not use the blunt tip vial access needle to inject TAKHZYRO as this may cause harm such as pain and bleeding.
3.7 Screw the 27 gauge ½-inch pointed tip injection needle to the syringe.
Important: Do not remove the needle cap from the needle when attaching to the syringe.
4. Select and prepare injection site
4.1 Choose a subcutaneous injection site on your stomach (abdomen), thigh, or upper arm.
It is important to rotate injection sites to keep skin healthy.
The area you choose for injection should be at least 5 cm (2 inches) away from any scars or your belly button (navel). Do not choose an area that is bruised, swollen, or painful.
The outer area of the upper arm is not recommended for self-injection.
4.2 Clean your injection site with an alcohol wipe and allow it to dry completely.
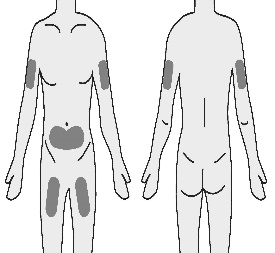
5. Inject TAKHZYRO
5.1 Pull off the needle cap straight away from the syringe without touching the needle. Do not pull on the plunger. Do not touch the needle tip or allow it to touch any other surface.
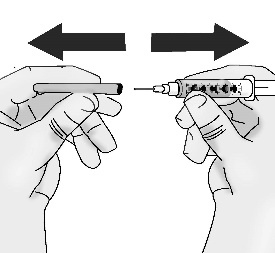
Important: TAKHZYRO should be administered within 2 hours of preparing the dosing syringe at room temperature. After the dosing syringe is prepared, it can be refrigerated at 2°C to 8°C and must be used within 8 hours.
5.2 Gently pinch about 2 cm (1 inch) of skin at your cleaned injection site and insert the needle.
Important: Be sure to inject into a subcutaneous space which is not too shallow (skin layer) or too deep (muscle).
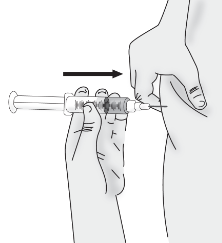
5.3 Push the plunger slowly until no contents remain in the syringe. Release the skin fold and gently remove the needle. Do not recap the needle.
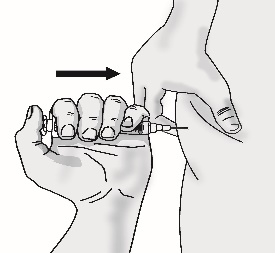
5.4 Discard the 27 gauge ½-inch pointed tip injection needle and the syringe in the sharps disposal container.
Source: Read Full Article

