Targeting bicarbonate in cancer
Bicarbonate ions are required for cell growth in some cancers, according to a Northwestern Medicine study published in the journal Molecular Cell.
Inhibition of a key bicarbonate transporter could lead to a cancer therapy with fewer side effects compared to currently available treatments, according to Issam Ben-Sahra, Ph.D., assistant professor of Biochemistry and Molecular Genetics and senior author of the study.
“This transporter is very important in cancer but less so in normal cells,” said Ben-Sahra, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “It could be a very interesting target.”
Eunus Ali, Ph.D., a postdoctoral fellow in the Ben-Sahra laboratory, was lead author of the study.
Bicarbonate ions are present in high concentration throughout the blood and are important for maintaining pH in cells. The ions are also used to make purine and pyrimidines, essential building blocks of nucleotide molecules.
In previously published studies, the Ben-Sahra laboratory found that many cancers upregulate nucleotide biosynthesis through the mTORC1 pathway, helping fuel cell growth and cancer proliferation. However, whether bicarbonate is controlled and utilized in this pathway was unknown, according to Ben-Sahra.
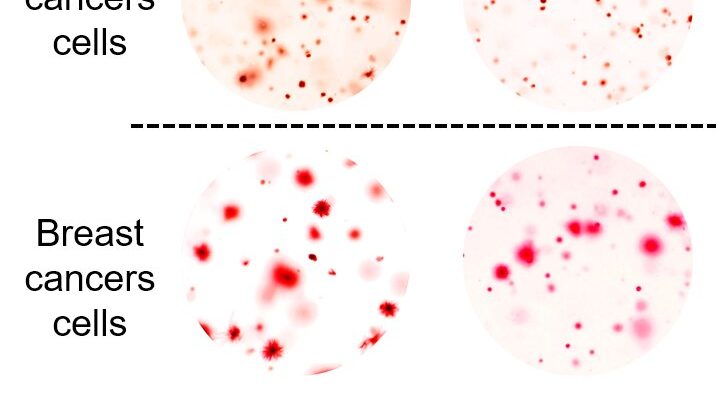
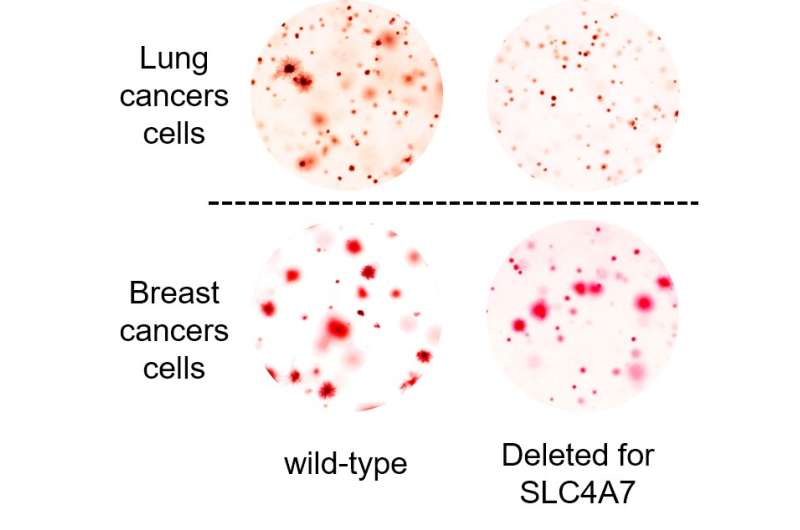
In the current study, Ben-Sahra and his collaborators examined usage of bicarbonate in normal and cancerous cells, using metabolic profiling to analyze bicarbonate usage at several stages. The investigators found that most bicarbonate transporters have little to do with mTORC1-related alterations in nucleotide metabolism which are triggered by cancer. However, one bicarbonate transporter, called SLC4A7, was upregulated in cancer cells as part of the mTORC1 pathway.
“Normal cells face high levels of bicarbonate in the blood, but in the microenvironment of tumors, you can have fewer nutrients,” Ben-Sahra said. “Here, bicarbonate can become limiting for tumor growth, necessitating usage of this transporter.”
The Ben-Sahra laboratory has explored several strategies to interrupt the metabolic upregulation incurred by cancer-related changes in mTORC1. Inhibiting SLC4A7 is among the most promising, Ben-Sahra said, because of its singular usage in cancer contexts.
“Targeting this one transporter will not affect pH or physiology of normal cells, because the other bicarbonate transporters can make up for it,” Ben-Sahra said. “But in the context of cancer, mTORC1 stimulates SLC4A7 transporter and we think this can be interrupted.”
Source: Read Full Article