What is Autophagy?
The word autophagy is derived from Greek words “auto” meaning self and “phagy” meaning eating. Autophagy is a normal physiological process in the body that deals with destruction of cells in the body.
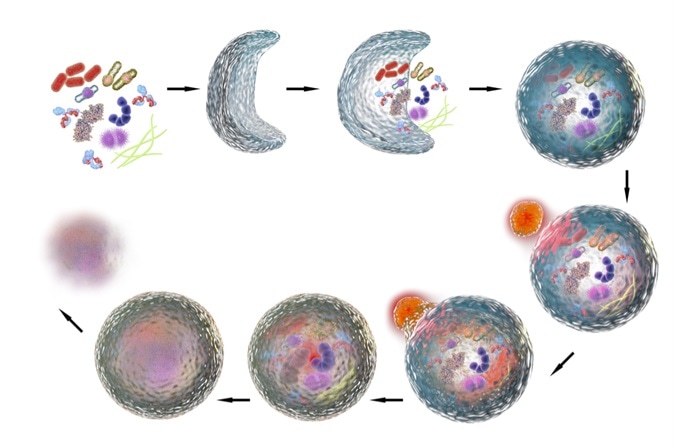
It maintains homeostasis or normal functioning by protein degradation and turnover of the destroyed cell organelles for new cell formation.
During cellular stress the process of Autophagy is upscaled and increased. Cellular stress is caused when there is deprivation of nutrients and/or growth factors.
Thus Autophagy may provide an alternate source of intracellular building blocks and substrates that may generate energy to enable continuous cell survival.
Autophagy and cell death
Autophagy also kills the cells under certain conditions. These are form of programmed cell death (PCD) and are called autophagic cell death. Programmed cell death is commonly termed apoptosis.
Autophagy is termed a nonapoptotic programmed cell death with different pathways and mediators from apoptosis.
Autophagy mainly maintains a balance between manufacture of cellular components and break down of damaged or unnecessary organelles and other cellular constituents.
There are some major degradative pathways that include proteasome that involves breaking down of most short-lived proteins.
Autophagy and stress
Autophagy enables cells to survive stress from the external environment like nutrient deprivation and also allows them to withstand internal stresses like accumulation of damaged organelles and pathogen or infective organism invasion.
Autophagy is seen in all eukaryotic systems including fungi, plants, slime mold, nematodes, fruit flies and insects, rodents (laboratory mice and rats), humans.
Types of autophagy
There are several types of Autophagy. These are:-
- Microautophagy – in this process the cytosolic components are directly taken up by the lysosome itself through the lysosomal membrane.
- Macroautophagy – this involves delivery of cytoplasmic cargo to the lysosome through the intermediary of a double membrane-bound vesicle. This is called an autophagosome that fuses with the lysosome to form an autolysosome.
- Chaperone-mediated autophagy – in this process the targeted proteins are translocated across the lysosomal membrane in a complex with chaperone proteins (such as Hsc-70).
- Micro- and macropexophagy
- Piecemeal microautophagy of the nucleus
- Cytoplasm-to-vacuole targeting (Cvt) pathway
Macro-autophagy
This method uses a double membrane-bound vesicle to deliver the cytoplasmic cargo to the lysosome. This double membrane-bound vesicle is called autolysosome, and it fuses with the lysosome.
Micro-autophagy
In this method, the components of cytoplasm are directly taken by the lysosome by invagination of the lysosomal membrane. In both macro- and micro-autophagy, large structures can be engulfed using specific and non-specific mechanisms.
Chaperone-mediated autophagy
In this method, the targeted proteins form a complex with chaperone proteins and then translocate across the lysosomal membrane. This complex is then recognized by lysosomal membrane receptor lysosomal-associated membrane protein 2A, leading to unfolding the degradation of the complex.
Selective autophagy
In certain cases, autophagy may be employed for selective organelles. For example, mitophagy is autophagy-dependent degradation of mitochondria. This function is important to preserve the integrity of these organelles and to limit the reactive oxygen species that is produced by the mitochondria.
Uth1p is a yeast protein and was the first protein to be identified in the process of mitophagy. However, it is still unclear how this protein interacts with autophagosome. Apart from this protein, other proteins that have been recently discovered in this process are Atg32, Atg8, Atg11, BNIP3L, Ulk-1, Parkin etc.
Peroxisomes and ribosomes can also be selectively eliminated via autophagy. For selective autophagy of peroxisomes, yeasts use a method where the vacuole directly engulfs the cargo or a process where the cargo is delivered to the vacuole via autophagosome.
Ribosomes are selectively removed by starvation or ribophagy. This process is dependent on the catalytic activity of a ubiquitin protease, Ubp3p/Bre5p.
Signaling pathways involved in autophagy
Rapamycin kinase (TOR kinase) acts as a signaling control point downstream of growth factor, hypoxia, ATP, and insulin signaling pathways. Akt kinase, PI3-kinase and growth factor receptors can activate TOR kinase when the nutrients are available, and this can promote growth and protein translation.
Hypoxia can also activate autophagy, by either hypoxia-inducable factor (HIF) pathway or HIF independent pathways. Hypoxia can promote ER stress, and mitochondria also have reduced function when the cell is in a hypoxic state. Thus, selective autophagy can help in eliminating ER and mitochondria when sufficient oxygen is not available.
Autophagy can also lead to the arrest of the cell cycle, although it is not clear if this process is dependent or independent of the TOR signaling.
Sources
- http://www.cellsignal.com/reference/pathway/pdfs/Autophagy.pdf
- http://www.wiley-vch.de/books/sample/3527314504_c01.pdf
- myweb.sabanciuniv.edu/…/curr-top-in-devel-review2.pdf
- http://morelife.org/references/full_papers/16973211.pdf
- users.unimi.it/…/Lecture%20Autophagy-%20pdf.pdf
- xa.yimg.com/…/Anita's+1st+Article.pdf
Further Reading
- All Autophagy Content
- Autophagy Process
- Autophagy Functions
- Autophagy Regulation
- Autophagy Signaling and Cellular Pathways
Last Updated: Jun 5, 2019

Written by
Dr. Ananya Mandal
Dr. Ananya Mandal is a doctor by profession, lecturer by vocation and a medical writer by passion. She specialized in Clinical Pharmacology after her bachelor's (MBBS). For her, health communication is not just writing complicated reviews for professionals but making medical knowledge understandable and available to the general public as well.
Source: Read Full Article
